Automatic Aseptic Multi-in-one Filling Line
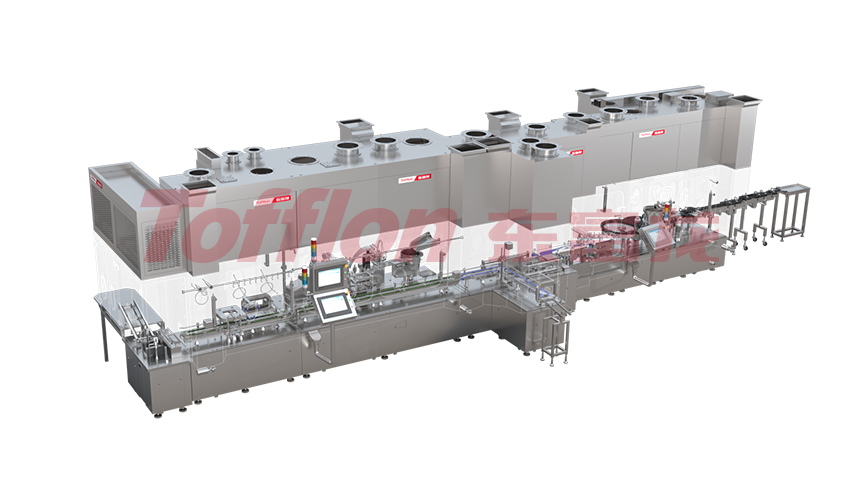
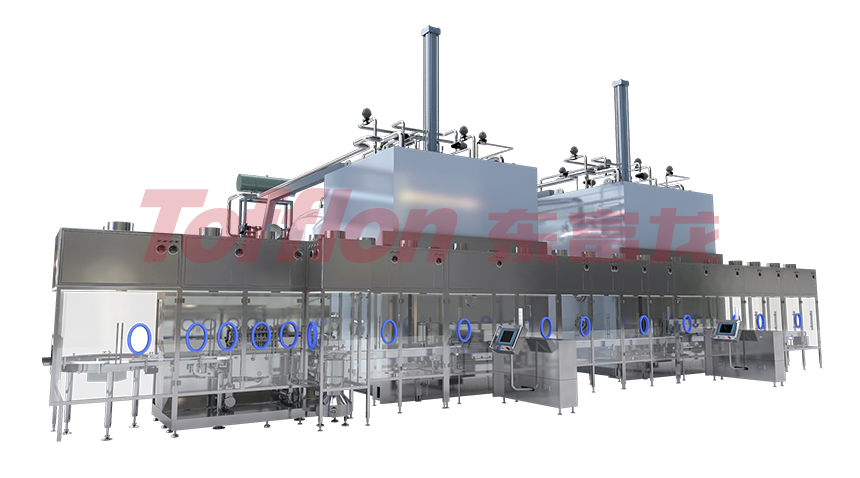
The automatic aseptic multi-in-one filling line processes three packaging materials: pre-filled syringes, vials, and cartridge vials. They're ready-to-use without cleaning and sterilization. It's suitable for commercial production with different yields like pilot-scale, medium-speed, and high-speed. The line's characteristic is flexible production. Via flexible mold conversion, it supports filling these materials, accelerating drug production safely and flexibly. It offers a low-risk, flexible processing method for aseptic packages, enhancing production efficiency and quality while reducing risks by adapting to various demands.
- The design complies with cGMP, FDA, and EU-GMP.
- Modular and compact design, easy to expand.
- Low investment in the project and short construction period.
- High equipment safety and strong stability.
- Can take into account all RTU packaging materials.
- Multiple filling doses and filling methods are available for selection.
- Integrated control system: highly integrated production data, safety interlocks, and full-process recording.
- The software design meets 21 CFR Part 11 and GAMP 5.
- Can be integrated with an Isolator full isolation system with environmental monitoring or an ORABS system.

-
Vaccines
-
Small - molecule drugs
-
Aesthetic medicine
-
Personalized treatment
-
Gene therapy
-
mRNA
-
Clinical trials
-
CDMO (Contract Development and Manufacturing Organization) business