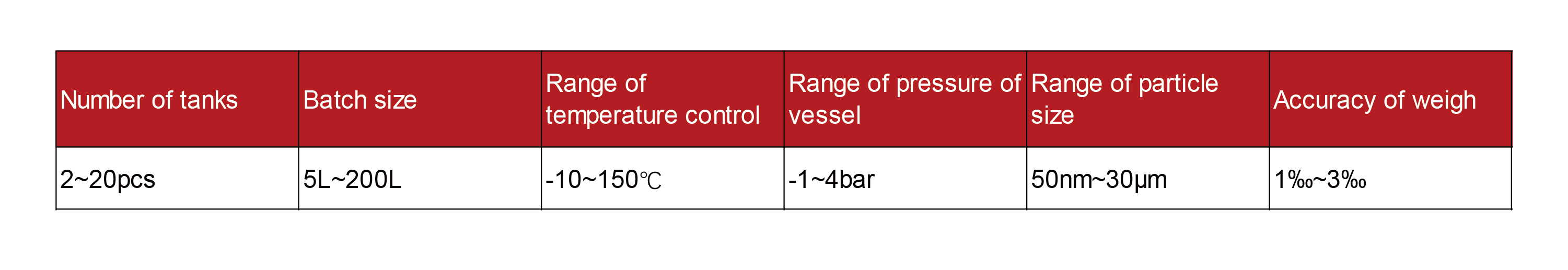
Formulation System of Liposome
The liposome formulation system is integrated automatic system which after the mixing of API and accessories to form liposome by top-down or bottom-up nanotechnology and aseptic production process. The system contains the raw material preparation unit, hydration unit, ultrafiltration unit, particle size reduce unit, API loading unit and sterile filtration unit. The formulation system is automatically one-touch operation system and the critical process parameters can be controlled and recorded to guarantee high reproducibility and stability. The formulation system is customized and modular design, which can satisfy most of the marketed liposome product of oversea. The system also can be customized for the R&D for drug-carrying liposome and blank liposome, not limited to active drug loading liposome and passive drug liposome. Many liposomes fabrication process can be satisfied in our system, such as film-hydration method, double-emulsification method, solvent injection techniques and in-situ preparation of liposomes. Tofflon can provide extruder, ultrafiltration, evaporator, isolator, filling machine, and freeze dryer to fabricate complete liposome production line.
- Overall layout solution: integrate plant layout, equipment, process and operation to optimization the material flow and personal flow of the layout.
- Critical process parameter control: extrusion pressure control, temperature control and pressure control.
- Sterility assurance: leakage test of tanks and pipeline to avoid leakage; Product sterile transfer and received by isolator.
- Residue control: optimized the process layout to minimize residue and improve product yield.

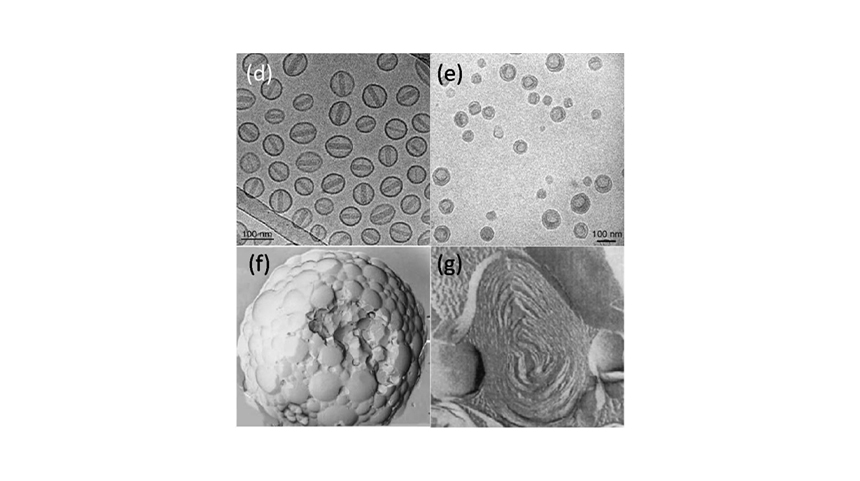
-
Satisfying commercial production of Doxil, Ambisome, DaunoXome, Onivyde, Arikaye, Vyxeos, Exparel and ect.