EPC Validation Management Services
Tofflon provides you with comprehensive EPC validation management services tailored to your sustainable development, with extensive practical experience in CQV management services for new construction/renovation of factories. Tofflon's validation management services integrate GEP with GMP management and resources organically, leveraging rich project practices. Through a full-cycle project validation management strategy, team, and resource matching and planning, they achieve project goals that are fast in delivery, high in quality, and compliant, thus facilitating the commercialization process of the project.
- Professionalism:Rich management practice in CQV for new construction/renovation of factories, possessing a scientific, comprehensive, and project-practice-verified validation management system that is fully organically integrated with product quality, ensuring professional and reliable validation results.
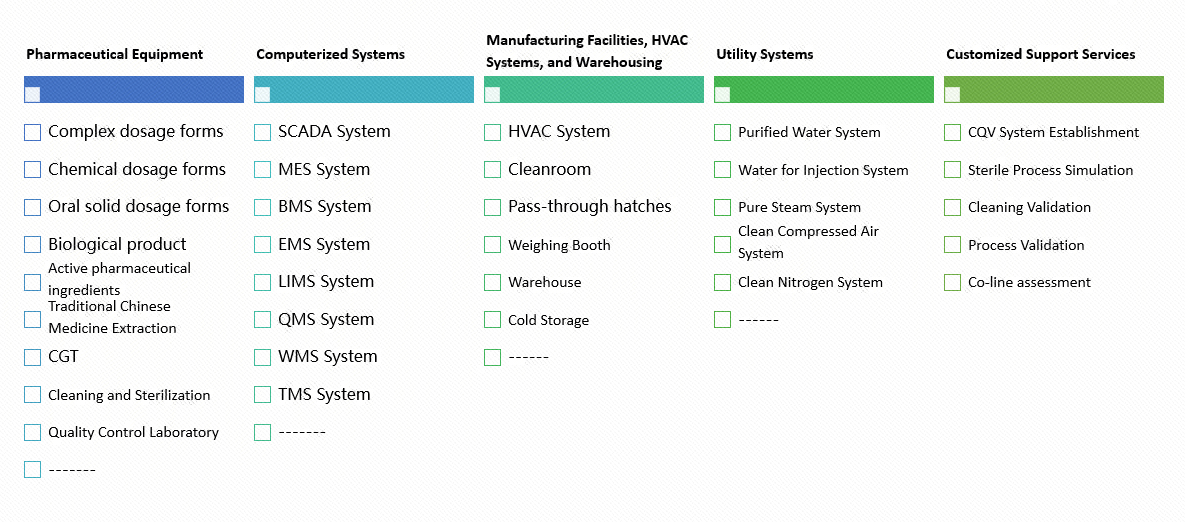
- Uniqueness:Owning autonomous products and engineering capabilities covering major process unit equipment, auxiliary unit equipment, and utilities in complex formulations, chemical preparations, biological products, oral solid preparations, APIs, and cell and gene therapy fields, we have a deeper understanding of system equipment and engineering, providing more advantageous services.
- Efficiency:A comprehensive project database allows for flexible access to long-accumulated archive resources of validation performance, achieving efficient and compliant delivery of project validation services.
- Scientificality:Based on current ISPE C&Q and GAMP5 guidance, applying ICH Q9 quality risk management concepts, and ICH Q8 with product and process risk at the core, we meet project and regulatory requirements.
- Reliability:Adhering to pharmaceutical industry regulations and supervisory requirements, referring to relevant guidelines, and tracking the development of cutting-edge industry technologies, we provide comprehensive validation and GMP compliance consulting integrated services, achieving a perfect combination of theory and practice.
- Capability:A project validation management team with rich practical experience and pharmaceutical company work background fully understands enterprise needs, achieving a scientific combination of validation plans with engineering project plans, ensuring the goal of efficient, high-quality, and compliant project delivery.


-
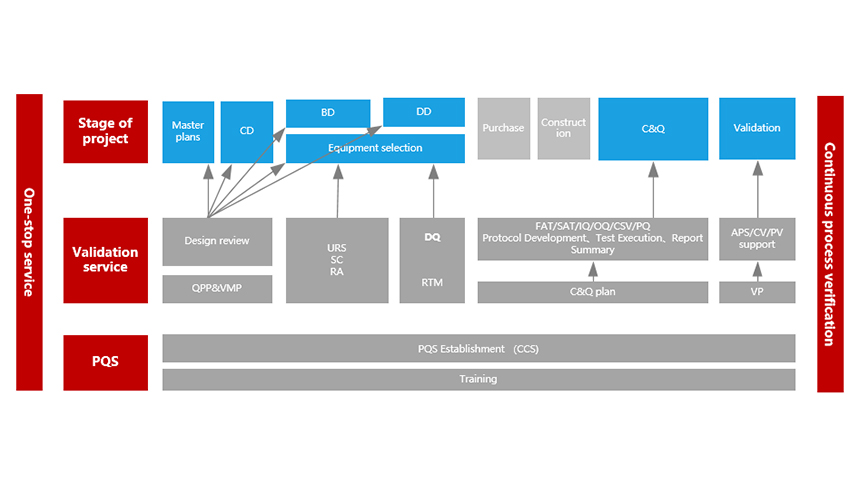
CQV system establishment or enhancement.
-
GMP scope validation strategy planning, confirmation, and training in the early stages of the project.
-
Identification and definition of multi-party responsibilities within the project validation management team.
-
Identification, confirmation, and coordination of project validation instruments and consumables.
-
Clarification and confirmation of validation documentation strategy, planning, confirmation, and coordination of work communication processes.
-
Drafting, review plan, circulation, and advancement of validation documents.
-
Planning and monitoring of project GMP validation plans, review, support, and coordination of supplier GMP confirmation services.
-
Training and strong supervision of test execution, confirming compliance, completeness, and effectiveness.
-
Management and coordination of deviations/changes/training/metrology, and delivery strategy and management of project validation documents.
-
Covering a range of services from URS, VMP to PQ, including Risk Assessment in Shared Facilities, APS, process/cleaning validation, etc.