Single-use Biological Swinger
The single-use biological swinger (SUR) is designed for cell therapy specially. The system provides good mixing and ventilation through rocking technology. The system ensures a sterile environment and reduces the risk of contamination through disposable biological bags. As a general-purpose cell culture and fermentation technology platform, the system provides reliable and accurate performance throughout the research, process development and production environment. Batch culture mode or perfusion culture mode can be used to obtain more cells and higher expression quantity, which is convenient for cell amplification culture.
- 1.Suitable for almost all the cell culture applications.
- 2.Suitable for different culture strategy, like batch culture, fed-batch culture and perfusion culture.
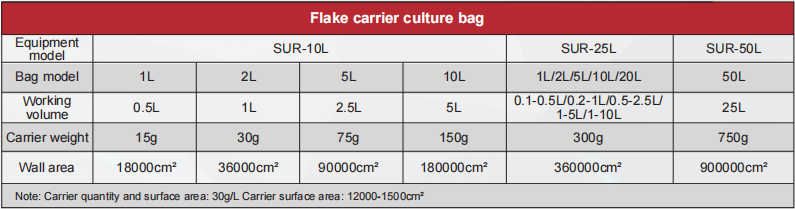
- 3.Suitable for suspension culture, flake carrier culture and micro carrier culture.
- 4.The disposable bio-reaction bag is a enclosed system that could minimize the risk of cell contamination.
- 5.Process monitoring: The key culture parameters (Swing speed, Angle, Temperature, DO, pH, perfusion rate, CO2 concentration, weight, pressure, etc.) could be precisely controlled by PID and tracked.
- 6.Two monitoring model applicable, CO2 concentration control model suitable for basic disposable bag, and pH/DO control model suitable for pH/DO applied disposable bag.
- 7.Suitable for use in a regulatory environment and complies with requirements and standards of cGMP.
- 8.Optional parts like pH/DO sensor module.
- 9.The optional remote monitoring of culture situation within the equipment can be monitored in real time through the mobile phone, message and E-mail, and the real-time alarm information and parameter information can also be checked.

-
1.Immune cell therapy (CAR-T, primary T-lymphocytes, etc.)
-
2.Stem cell preparation (cord blood stem cells, embryonic stem cells, etc.)
-
3.Preparation of antibody drugs (PD-1, PDL-1, etc.)
-
4.Preparation of recombinant protein products (recombinant coagulation factor, EPO, TPO, etc.)
-
5.Preparation of viral vectors (ADV, oncolytic virus, etc.)
-
6.Vaccine preparation (ADV vaccine, mRNA vaccine, etc.)
-
7.Laboratory process development and exploration